PRODUCT IDENTIFICATION
27164-46-1(Sodium)
H.S. CODE
TOXICITY
CLASSIFICATION
PHYSICAL AND CHEMICAL PROPERTIES
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
APPLICATIONS
APPEARANCE
ASSAY (HPLC)
20ppm max
PH
INDIVIDUAL IMPURITY
1.0% max
5kgs - 25kgs
|
|
|
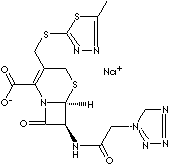
| CEFAZOLIN SODIUM | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 25953-19-9
(Base) 27164-46-1(Sodium) |
|
| EINECS NO. | 247-362-8, 248-278-4 | |
| FORMULA | C14H13N8NaO4S3 | |
| MOL WT. | 476.48 | |
|
H.S. CODE |
||
|
TOXICITY |
||
| SYNONYMS | Ancef; Kefzol; Zolicef; | |
| (6R,7R)-3-[(5-methyl-1,3,4-thiadiazol-2- yl) thiomethyl]-8-oxo-7-[2-(1H-tetrazol-1- yl) acetamido]-5-thia-1-azabicyclo[4.2.0] oct-2-ene-2-carboxylate, monosodium; sodium (6R-trans)-3-[[(5-methyl-1,3,4-thiadiazol-2-yl)thio] methyl]-8-oxo-7-(1H-tetrazol- 1-ylacetamido)-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate; | ||
|
CLASSIFICATION |
||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | White to off-white powder | |
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY | ||
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY |
| |
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
||
|
REFRACTIVE INDEX |
||
| FLASH POINT |
| |
| STABILITY | Stable under ordinary conditions. | |
|
APPLICATIONS |
||
| 1ST GENERATION CEPHALOSPORIN, Inhibiting Cell Wall Biosynthesis. | ||
| SALES SPECIFICATION (USP, BP, Ph.Eur) | ||
|
APPEARANCE |
White to off-white powder | |
|
ASSAY (HPLC) |
96.0 - 101.0% | |
| OPTICAL ROTATION | -24° ~ -15° | |
| SOLUTION CLARITY | clear (in water) | |
| N,N-DIMETHYLANILINE |
20ppm max |
|
| WATER ((K.F) | 3.0% max | |
|
PH |
4.0 - 6.0 | |
|
INDIVIDUAL IMPURITY |
1.0% max |
|
| STERILITY | sterile | |
| BACTERIAL ENDOTOXINS | 0.15 EU/mg max | |
| TRANSPORTATION | ||
| PACKING | 5kgs - 25kgs | |
| HAZARD CLASS | ||
| UN NO. | ||
| OTHER INFORMATION | ||
|
|
|